The Relation of Characteristics and Biogeographic History of Antarctic Notothenioid Fish to Adaptive Radiation
Literature Review
The Relation of Characteristics and Biogeographic History of Antarctic Notothenioid Fish to Adaptive Radiation
by Laura Goetz (for Advanced Writing for the Sciences course, 2016)
Abstract
Notothenioids are a diverse suborder of fish that dominate the Southern Ocean waters surrounding Antarctica and South America. They are characterized based on morphology, hunting strategies, antifreeze glycoproteins, buoyancy and oxygen transport. These characteristics combined with the biogeographical history of notothenioids and the formation of Antarctica and its geographic barriers illustrate one of the strongest examples of adaptive radiation in marine habitats. This paper intends to review each of these characteristics and analyze the current knowledge of adaptive radiation along with the geographic history of Antarctica.
Introduction
In 1954, Johan Ruud wrote an article for Nature about the bloodless fish in Antarctica (Prisco et al. 2002). Icefish, family Channichthyidae from suborder Notothenioidei, look similar to pikes in shape but are scale-less and when cut open, their blood and gills are transparent or white. This family and its relative families have become key in investigate a variety of different ecological and molecular processes, like adaptive radiation and genome evolution. Notothenioid fish are divided into five families: Nototheniidae (cod icefishes), Bathydraconidae (dragonfish), Channichthyidae (white-blooded fish), Artedidracondiae (barbed plunderfish), and Harpagiferidae (Rutschmann et al. 2011). The ones used most often for comparison are dragonfish from Bathydraconidae and white-blooded fish from family Channichthyidae because of the larger number of species in the families and the distinct characteristics of each family (Near et al. 2006). Main distinguishing characteristics that divide these fish into these families are antifreeze glycoproteins (AFGPs), buoyancy and oxygen transportation methods.
AFGPs evolved from protease proteins in response to the cooling waters of Antarctica (Cheng 1998, Hellman et al. 2012, and Cziko et al. 2014). They are present in most notothenioids and keep the fish alive by binding to ice crystals to keep a stable body temperature (Cheng 1998, Hellman et al. 2012, and Cziko et al. 2014). AFGPs are used continuously in different parts of the notothenioid’s body and are constantly recycled while eliminating some internal ice (Cheng 1998, Hellman et al. 2012, and Cziko et al. 2014). They do not, however, eliminate all internal ice, causing potential dangers for the organism.
Notothenioids were originally benthic but evolved to dominate the water column and seafloor by increasing buoyancy. This movement allowed for diversification of feeding habits and easing pressure off competition between species although energetically, it is not favorable.
Another key differentiating characteristic of notothenioids is oxygen transport. Channichthyids have evolved to not using hemoglobin to transport oxygen throughout the body like its relatives. The family evolved to compensate but the adaptation is seemingly unfavorable with current research.
Adaptive radiation is the ecological phenomenon that explains the fast branching of species from an ancestor in order to move into open niches. Evidence of adaptive radiation includes common ancestry of the species under investigation, early bursts of branching that slow down with time and a correlation between phenotype and environment (Matshiner et al. 2015). Notothenioids exhibit these characteristics. These fish were benthic for millions of years until modifications in swim bladders and other physiological changes allowed the notothenioids to also move into the water column (Rutschmann et al. 2011, Fernandez et al. 2012 and Wilson et al. 2013). The notothenioids also fit the species flock concept: a term describing the clustering of many closely related species flocking together (Eastman 2004 and Matshiner et al. 2015). A species flock is indicative of adaptive radiation occurring so the key characteristics of a species flock is similar to that of adaptive radiation. These characteristics are species richness, common ancestry, geographic territory and high amount of existence in a certain region (Matshiner et al. 2015).
Section 1: Characteristics and Diversity of Icefish
Although notothenioids have a common ancestor, the suborder experiences high levels of diversity among families indigenous to the Southern oceans surrounding the tip of South America and the shores of Antarctica. Using AFGPs to keep from freezing, they dominate the diversity and biomass of their niches and play large parts in their surrounding food webs because of the diversity between and within families as well as the geographic isolation. Channichthyids are the longest of the Notothenioid fish and have long thin bodies with thicker heads (Eastman 2004). They have large mouths, moderate sized eyes, and skin rather than scales (Figure 1). Bathydraconids and artedidraconids are the most benthic families (Rutschmann et al. 2011). Artedidraconids have large eyes, thin bodies with spines while bathydraconids have no spines, large eyes, thin bodies and large mouths (Figure 1) Nototheniids have large rounded heads, moderate sized eyes, large mouths with lips and resemble cod (Figure 1). Harpagiferidae only has genus Harpagifer (Figure 1). These fish have rounded heads, small mouths and small eyes (Figure 1). Nototheniids are the most diverse family: different species employ plankton, nekton and/or benthos feeding (Rutschmann et al. 2011). Channichthyids prey on a variety of organisms like krill and fish on both the floor of the ocean and the water column (Eastman 2004 and Rutschmann et al. 2011). Both bathydraconids and artedidraconids feed by ambushing prey like small fish and plankton (Rutschmann et al. 2011).
The morphological data of notothenioids have been used to study phylogeny by comparing their unusual combinations of characteristics such as having 3 pectoral radials, poor quality/lacking pleural ribs, one nostril on each side of the head, non-pungent fin spines, no swim bladder, 2-3 lateral lines, jugular pelvic fins, nasal accessory organs (Matshiner et al. 2011, Fernandez et al. 2012, and Near et al. 2015). These states are used to compare clades but the optimal data comes from combining morphological and genetic datasets.
Figure 1- Cladogram for suborder notothenioids based on maximum likelihood model from Near et al (2012) showing the differences in physical characteristics among families. The study based the phylogeny on five nuclear and two mitochondrial genes. The non-Antarctic sister lineages are not highlighted at the top of the figure. The family Nototheniidae is blue, Harpagiferidae is red, Artedraconidae is green and Channichthyidae is pink (figure from Eastman et al. 2014).
The most debate on the topic of notothenioids is the identification of the sister lineage to the clade. Three possible sister lineages had been previously identified: Bovichtidae,Pseudaphritis urvillii, and Eleginops maclovinus (Near et al. 2015). A recent study was able to successfully identify Percophis brasiliensis as the sister lineage of notothenioids by using a maximum likelihood method that combines geography of a species, phylogenetic relationships, divergence times and paleogeographic history of more than 650 species to find distinguishable clades (Near et al. 2015). The identification led to accurate clade groupings and names. With this methodology, P. brasiliensis within family Percophidae was identified as the sister lineage with strong support (Near et al. 2015). E. maclovinus is the next species closest to the Cryonotothenioidea clade, with Bovichtidae and P.urvillii the next closest to the clade Eleginosioidea (Near et al. 2015). The identification of the sister lineage allows for future deeper investigation of the processes of adaptive radiation and placement of other lineages.
Section 1.1: Antifreeze Glycoproteins (AFGPs)
Antarctic icefish are at high risks of death from blood and other body fluids freezing from the constant freezing temperatures of their environment. They encounter ice and consume ice in the form of anchor ice, the surface of the ocean and also when ingesting food (Cziko et al. 2014). The crystals move into multiple organs of their bodies. This includes the gills, digestive tract and the spleen (Cziko et al. 2014 and Matshiner 2015). In response, AFGPs have been developed from a pancreatic trypsinogen gene to survive in the extreme temperatures without body fluids freezing (Matshiner 2015). This development allowed for the movement of notothenioids into the subzero waters of Antarctica (Eastman 2004 and Matshiner 2015). AFGPs are the most important adaptation for icefish in terms of speciation.
AFGPs are three amino acids repeated, Alanine-Alanine-Threonine tripeptides linked to galactose-N-acetylgalactosamine (Cheng 1998). AFGPs identify ice inside the body then permanently bind to these crystals, keeping the body temperature at a stable temperature (Cziko et al. 2014). AFGPs are created in the pancreas and are released into the digestion tract to wrap around ice crystals so that they can be excreted safely as waste through the anus with excrement without growing and killing the fish (Evans et al. 2012 and Matshiner et al. 2015). The AFGPs that are not used are reabsorbed into the epithelium in the rectum and enter the bloodstream to circle back to the liver to be released later (Evans et al. 2012 and Matshiner et al. 2015). This combined with AFGPs on the surface of the body keep the body temperature of the fish stable (Evans et al. 2012 and Matshiner et al. 2015). Not all ice is eliminated from the fish’s system, leading to the hypothesis that summer melting aids with elimination of ice from the body (Evans et al. 2012 and Matshiner et al. 2015). Research found that it does not reliably melt internal ice, leading to a gap in knowledge (Cziko et al. 2014).
Figure 2– Illustration of simple cladogram for four different characteristics of notothenioids and how AFGPs function. (a) A cladogram based on loss of swim bladder, loss of heat shock proteins, evolution of AFGPs and loss of hemoglobin. The cladogram shows that the loss of AFGPs contributed to the branching of Antarctic icefish from other notothenioids. (b) The process of AFGPs containing ice in the body of a fish. Ice that is consumed in diet is contained by AFGPs from the liver in the digestive tract. AFGPs also attack ice crystals on the skin. (figure from Matshiner et al. 2015).
All Antarctic icefish possess AFGPs to survive, showing that AFGPs are not only highly conserved among species with relatively few mutations, but that AFGPs aided notothenioids to survive the mass extinctions that led to the opening of niches (Matshiner et al. 2011 and Matshiner et al. 2015). Other adaptations in morphology, like changes in buoyancy, allowed notothenioids to move into other niches.
Section 1.2: Buoyancy
Buoyancy has two components, one passive (static life) and one active (dynamic lift) to keep a fish off the seafloor (Fernandez et al. 2012). Static lift describes the difference between weight in air versus weight in water; dynamic life movements and activity of the fish (Fernandez et al. 2012). One of the most cited examples of notothenioids domination is their pelagization to utilize other niches by evolving from a negative buoyancy to a close to neutral buoyancy (Fernandez et al. 2012 and Wilson et al. 2013). They evolved from a common benthic ancestor to colonize other marine habitats like epibenthic, semipelagic, cryopelagic and pelagic niches (Rutschmann et al. 2011). Unlike other pelagic fish, notothenioids do not have swim bladders (Rutschmann et al. 2011, Fernandez et al. 2012, and Near et al. 2012). Without a swim bladder, notothenioids need to support approximately 5% of their weight in the air when swimming in the water to remain buoyant (Fernandez et al. 2012).
Notothenioids required three main different adaptations. These adaptations include reduced ossification of the spinal column, deposited lipids in large amounts of adipose cells, and demineralized scales in some species (Fernandez et al. 2012 and Matshiner et al. 2015). These adaptations decrease the energy requirements of remaining neutrally buoyant but not entirely. Because neutral buoyancy in notothenioids is rare, it still expends energy to stay pelagic (Fernandez et al. 2012). With more comparison of buoyancy to behaviors, it has become established that notothenioids need control over buoyancy for nesting (Fernandez et al. 2012).
Section 1.3: Hemoglobin Loss
Hemoglobin is sensitive to partial pressure of oxygen, temperature and the structure in each species reflects what kind of environment they were once subject to, making it an excellent measurement of speciation (Prisco et al. 2007). Icefish have back evolved to not use hemoglobin for oxygen transport unlike other fish and instead use no protein binding (Prisco et al. 2007). To adapt, white-blooded fish evolved to improve heart output, increase blood volume, increase uptake of oxygen and also cut metabolic rates (Near et al. 2006). With a variety of species to compare and molecular technology progressing, the molecular processes that have formed the white-blooded fish can be isolated. (Prisco et al. 2007). Hemoglobin development genes in fish are divided into four different categories. The first two are juvenile and adult globin complexes (Prisco et al. 2002). The juvenile globin complexes aid in the development of hemoglobin and the adult globin complexes maintain hemoglobin production generally. The second two categories are α1-globin and β-globin (Prisco et al. 2002). These categories specify the location of the globin complex in question. The adult α1-globin complex, for example, comprises of the α1-globin gene linked to the β-globin gene so that the 5’ end of each gene is facing outwards with 4.3 kb of DNA separating the two genes (Figure 3; Prisco et al. 2002).
Figure 3– Illustration of alpha and beta globin complexes of icefish. (a) shows the different compositions of the complexes based on if the genes are truncated or not based on different species and the length of these genes. N.ionah, P.charcoti and N. angustata are all red blooded fish. The introns are colored red and the exons are colored green. The 14 icefishes only have a truncated alpha globin complex. (b) shows the hemoglobin results in a cladogram based on family. (figure from Near et al. 2006)
It was unknown for years if this back evolution occurred because of a single mutational event or if multiple events were needed for icefish to render hemoglobin useless. With the discovery that one icefish species, Neopagetopsis ionah had a nearly intact, but useless αβ-globin genes, it became clear that N.ionah is a transitional species, meaning that multiple events were needed (Near et al. 2006). In typical icefish without hemoglobin, the genes are fragmented into short clips of DNA that cannot code for any uses (Near et al. 2006). The N.ionah set is nearly intact except it had a single mutation that caused a splice that deletes the 10 base pairs coding for the CAG/G acceptor, causing the β-globin gene to lose its function (Near et al. 2006). The loss of hemoglobin is caused by the loss of the β-globin subunit gene from this single mutation (Matshiner et al. 2015).
There are two possible explanations for the loss of hemoglobin in icefish but neither has enough support to make a concrete conclusion. One theory is that because iron is a limited element in the oceans, the fish evolved to not need it for oxygen transportation (Heyden et al. 2012 and Matshiner et al. 2015). Another possible explanation is that fish eliminated the bulk of erythrocytes because the near freezing temperatures of the waters increase the viscosity of body fluids (Bargelloni et al. 1998). Both lack in-depth research.
Section 2: Antarctic Climate Changes and the Speciation of Notothenioidei
Antarctica’s Southern Ocean comprises of the coldest seawater temperatures on Earth. The water temperature is nearly constant at -1.86°C, while seawater freezes at -2°C (Matshiner et al. 2015). Evidence from the drill cores and sea isotope records indicate that this was not always the case (Matshiner et al. 2011). In the early Cenozoic era, Antarctica was a component of the landmass Gondwana and was surrounded by temperate waters (Bargelloni et al. 1998, Matshiner et al. 2011). Gondwana comprised of Africa, South America, Australia, and Antarctica (Near et al. 2015). A recent study found that the diversification of notothenioids follows the geographic changes in the Southern Ocean (Near et al. 2015). The cleavage of Antarctica from Gondwana prompted the formation of the Drake Passage 122 Ma, which now separates Antarctica and the tip of South America (Bargelloni et al. 1998, Cheng et al. 2008, Near et al. 2015).
The Weddellian Province comprised of Australia, Antarctica, South American and New Zealand in the late Cretaceous period (Near et al. 2015). The temperate, shallow seas were an optimal environment for supporting marine life and as a result, hosted a variety of species (Near et al. 2015). These seas hosted the ancestors of notothenioids. This hypothesis is supported by the presence of close relatives of notothenioids, like Pseudaphritis, Halaphritis and Bovichtus (Near et al. 2015). When comparing the branching pattern of speciation of Weddellian fish to the breakup of the landmasses comprising of the Weddelian province, a clear pattern emerges. As South America separated from Australia and Antarctica, Bovichitdae continued to inhabit South America while Pseudaphritiodea remained with the remaining landmass (Near et al. 2015). The disjunction of these species occurred an estimated 88.6 Ma (Near et al. 2015). Pseudaphristis continued to inhabit the coasts of Australia as the clade Elegniopsioidea remained with Antarctica and South America. The separation occurred 93.8 Ma, while the separation of Australia and Antarctica occurred an estimated 90 Ma (Near et al. 2015). The diversification of Eleginopsioidea then took place with the formation of the Drake Passage (Near et al. 2015). The separation of the Antarctic fish clade Cryonotothenioidea from Eleginops was completed as Antarctica separated fully from Antarctica (Near et al. 2015).
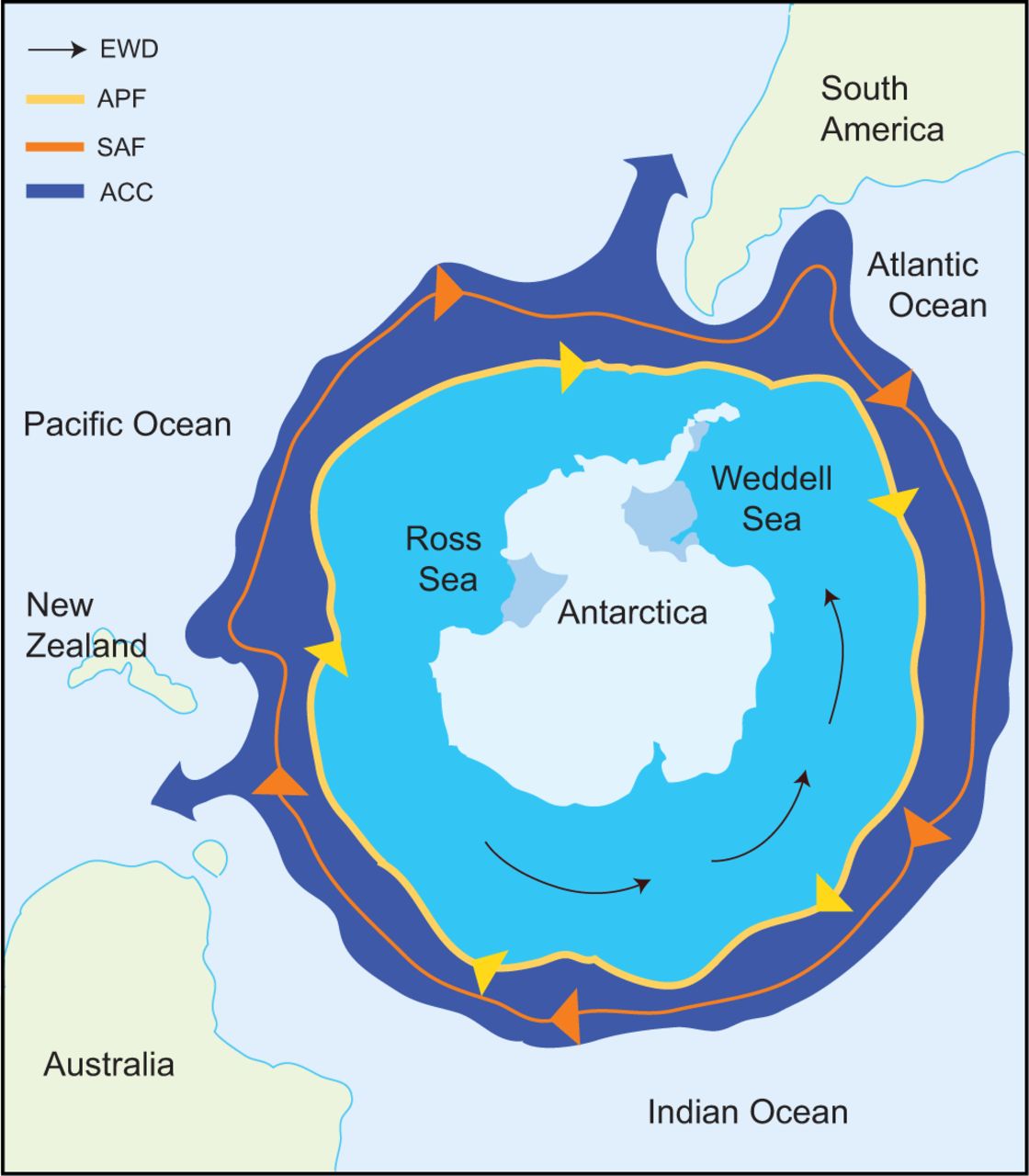
The formation of the Drake Passage led to the geographic isolation of Antarctica and also the formation of several natural phenomena that thermally isolates Antarctica. The Antarctic Circumpolar Current formed 22-25 Ma and is strong current that both geographically and thermally isolates the Antarctic shores (Figure 4; Bargelloni et al. 1998, Cheng et al. 2008, and Rutschmann et al. 2011). Coupled with the Antarctic Polar Front that also surrounds the continent, the marine life inhabiting the freezing waters of Antarctica are effectively isolated from other species of marine life (Figure 4). This isolation prompted the selective speciation of notothenioids.
Figure 4- Map depicting the geographic isolation of Antarctica. The Antarctic Polar Front (APF) hugs the Southern Ocean waters while moving in a clockwise direction with the Antarctica Circumpolar Current (ACC) immediately outside the APF. (figure from Beers and Jayasundara 2015).
Section 3: Adaptive Radiation of Notothenioids
The species diversity in the extremes of marine habitat is low compared with that of temperate or tropic waters due to the harshness of the polar environment. Fewer organisms can survive because fewer organisms can thrive in extreme environments (Eastman 2004). Notothenioid fish are well suited for survival after millions of years of evolution (Prisco et al. 2002). Although the species diversity is poor, the diversity from adaptive radiation within Notothenioid fishes is representative ecological processes such as adaptive radiation (Eastman 2004).
One aspect of study to confirm notothenioids underwent adaptive radiation is the confirmation of an early burst scenario (Matshiner et al. 2011, Wilson et al 2013, Colombo et al 2015, and Matshiner et al 2015). If adaptive radiation is the mechanism causing the branching of species, the most speciation should occur closely to the occurrence of the common ancestor. Multiple studies attempted to prove that notothenioids exhibit evidence of early bursts. Near et al. (2012) found high adaptive radiation much later than expected and no evidence of Miocene bursts using maximum likelihood methodology with phylogeny of five nuclear and two coding genes. It was concluded that the constant niches opening during glaciation led to the high Pleistocene diversity for radiation (Near et al. 2012).
Another study by Wilson et al. (2013) supported these results by comparing the opercles of different fish. Opercle shape is indirectly related to feeding tactics, which is directly related to position in the water column (Wilson et al. 2013). By comparing the shape of these organs, the geometric morphometrics can distinguish between clades (Wilson et al. 2013). No evidence of early bursts was found, yet a later peak similar to Near et al. 2012 was found. It was concluded that this peak was because of cycling of niches and repeated expansion of fish into different habitats (Wilson et al. 2013). These two studies lack the ability to detect the early bursts that formed the notothenioid clade. A later study used time calibrated phylogeny of 49 fish species and five traits of buoyancy and habitat characteristics and found clear evidence of early bursts by analyzing the relationship between phenotype and environment (Colombo et al. 2015).
AFGPs were used to attempt to determine early bursts but they do not fit into the timing of speciation (Matshiner et al. 2011, Near et al. 2012 and Colombo et al. 2015). One study attempted to link the adaptive radiation of notothenioids to the freezing of Antarctica’s glaciers by analyzing fossil-calibrated phylogeny of notothenioids and close relatives (Matshiner et al. 2011). The study found that notothenioids’ radiation began in between periods Oligocene and Miocene and that this occurred with the increasing amount of ice in Antarctica (Matshiner et al. 2011). It was then falsely concluded that radiation is triggered by AFGP development. Another study using a time calibrated molecular phylogeny discovered that the speciation of notothenioids occurred an estimated 10 Ma after AFGPs developed (Near et al. 2012). The study concludes that the radiation was not triggered by AFGPs but by AFGP protection from freezing and the movement into new niches with changes in buoyancy and feeding (Near et al. 2012). Later research found the adaptive radiation of notothenioids occurred approximately 13.4 Ma in the late Miocene period, which is much later than the development of AFGPs (Colombo et al. 2015).
These studies demonstrate the difficulty in reconstructing the evolutionary past of notothenioids by focusing on solely fossil, genome or morphological phylogeny. Studies that compared multiple levels in speciation for notothenioids were able to identify stronger support for adaptive radiation triggers than studies that focused on one level.
The theory of adaptive radiation predicts that radiation results in differentiated groups from a single common ancestor. The lack of distinct phases of adaptive radiation suggests that with time, these clades will become more differentiated from each other. More analysis is needed to conclude if adaptive radiation of notothenioids is continuing or not by comparing fossil morphological phylogeny with current morphology.
Conclusion
Past research has slowly pieced together the puzzle that is the speciation of Antarctic icefish. It is now known that the icefish were part of a rich environment until the drop of temperatures caused mass extinction. With the development of AFGPs from protease genes, the icefish were able to survive and eventually dominate the oceans with changes in oxygen transport systems and buoyancy. The geographic isolation that separated Cryonotothenioidea from larger clans is clearly correlated with the fragmentation of Gondwana.
These evolutionary and geographic events support the hypothesis that notothenioid diversity is caused by adaptive radiation. The early bursts caused by the change in preferences based on habitat show clear evidence of early bursts of speciation and diversification. Early bursts suggest that the radiation is moving in phases. The difficulty in interpreting the data supporting adaptive radiation suggests two different things: the studies are increasing in complexity and the adaptive radiation process has not been completed. With the progress of bioinformatics and computer’s abilities to process larger datasets with more speed, it will be easier for scientists to analyze multi-level information rather than two or three levels. The advancement will help untangle the radiation patterns and differentiate phases so the processes of the adaptive radiation can be understood. With understanding the process, research will be able to support the hypothesis that adaptive radiation is not complete.
Acknowledgements
Thank you so much to Colin Davis for listening to me stress out about this paper for weeks and for being one of the most supportive people in my life. Thank you to Sydney Juris for always listening to me and encouraging me whenever I’m feeling down. Thank you again to Kat O’Brien for being my friend and future peer in marine science research. Thank you to Professor Bill Detrich for accepting my application as a Antarctic field research team member. Also one more thank you to the people who have worked for years in this field to try and understand the amazing processes of these fish.
References
Bargelloni, L., Marcato, S., and Patarnello, T. (1998). Antarctic fish hemoglobins: Evidence for adaptive evolution at subzero temperature. Proceedings of the National Academy of Sciences 95, 8670-8675.
Beers, J. and Jayasundara, N. (2015). Antarctic notothenioids fish: what are the future consequences of ‘losses’ and ‘gains’ acquired during long-term evolution at cold and stable temperatures? Journal of Experimental Biology 218, 1834-1845.
Chen, Z., Cheng, C-H., Zhang, J., Cao, L., Chen, L., Zhou, L., Jin, Y., Y, Hua., Deng, C., Dai, Z., Xu, Q., Hu, P, Sun, S., Shen, Y., and Chen, L. (2008). Transcriptomic and genomic evolution under constant cold in Antarctic notothenioid fish. Proceedings of the National Academy of Sciences 105, 12944-12949.
Cheng, C. (1998). Evolution of the diverse antifreeze proteins. Current Opinion in Genetics & Development 8, 715-720.
Colombo, M., Damerau, M., Hanel, R., Salzburger, W., and Matschiner, M. (2015). Diversity and Disparity Through Time in Adaptive Radiation of Antarctic Notothenioid Fishes. The Journal of Evolutionary Biology 28, 376-394.
Cziko, P., DeVries, A., Evans, C., and Chen, C. (2014). Antifreeze protein-induced superheating of ice inside Antarctic notothenioids fishes inhibits melting during summer warming. Proceedings of the National Academy of Sciences 111, 14583-14588.
Evans, C., Hellman, L., Middleditch, M., Wojnar, J., Brimble, M and Devries, A. (2012). Synthesis and recycling of antifreeze glycoproteins in polar fishes. Antarctic Science 24, 259-268.
Eastman, J. (2004). The nature of the diversity of Antarctic fishes. Polar Biology 28, 93-107.
Eastman, J,. Witmer, L., Ridgely, R., and Kuhn, K. (2014). Divergence in Skeletal Mass and Bone Morphology in Antarctic Notothenioid Fishes. Journal of Morphology 275, 841-861.
Fernandez, D., Ceballos, S., Malanga, G., Boy, C., and Vanella, F. (2012). Buoyancy of sub-Antarctic notothenioids including the sister lineage of all other notothenioids (Bovichtidae). Polar Biology 35, 99-106.
Heyden, P., Roychoudhury, A., Mtshali, T., Tylisczczak, T., and Myneni, S. (2012). Chemically and Geographically Distinct Solid-Phase Iron Pools in the Southern Ocean.Science 338, 1199-1201.
Matschiner, M., Colombo, M., Damerau, M., Ceballos, S., Hanel, R., and Salzburger, W., (2015). The Adaptive Radiation of Notothenioid Fishes in the Waters of Antarctica.Extremophile Fishes, 35-57.
Matschiner, M., Hanel, R., and Salzburger, W. (2011). On the Origin and Trigger of the Notothenioid Adaptive Radiation. PLoS One 6.
Near, T., Parker, S., and Detrich, H. (2006). A Genomic Fossil Reveals Key Steps in Hemoglobin Loss by the Antarctic Icefishes. Molecular Biology and Evolution 23, 2008-2016.
Near, T., Dornburg, A., Kuhn, K., Eastman, J., Pennington, J., Patarnello, T., Zane, L., Fernandez, D., and Jones, C. (2012). Ancient climate change, antifreeze and the evolutionary diversification of Antarctic fishes. Proceedings of the National Academy of Sciences 109, 3434-3439.
Near, T., Dornburg, A., Harrington, R., Oliveira, C., Pietsch, T., Thacker, C., Satoh, T., Katayama, E., Wainwright, P., Eastman, J., and Beaulieu, J. (2015). Identification of the notothenioids sister lineage illuminates the biogeographic history of an Antarctic adaptive radiation. BMC Evolutionary Biology 109.
Prisco, G., Cocca, E., Parker, S., and Detrich, H., (2002). Tracking the Evolutionary Loss of Hemoglobin Expression by the White-Blooded Antarctic Icefishes. Gene 295, 185-191.
Prisco, G., Eastman, J., Giordano, D., Parisi, E., and Verde, C., (2007). Biogeography and adaptation of Notothenioid fish: Hemoglobin function and globin-gene evolution. Gene398, 143-155.
Rutschmann, S., Matschiner, M., Damerau, M., Muschick, M., Lehmann, M., Hanel, R., and Salzburger, W. (2011). Molecular Ecology 20, 4707-4721.
Wilson, L., Colombo, M., Hanel, R., Salzburder, W., and Sanchez-Villagra, M. (2013). Ecological disparity in an adaptive radiation: opercular bone shape and stable isotopes in Antarctic icefishes. Ecology and Evolution 9, 3166-3182.